ARES BBM
THE FIRST HALF-MASK FOR PROTECTION AGAINST BIOLOGICAL AGENTS.
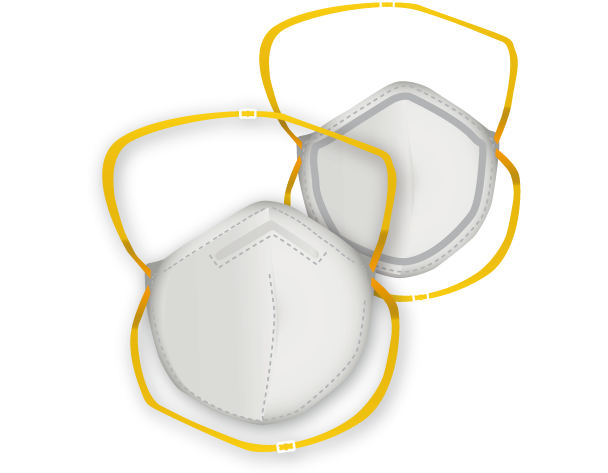
DISPOSABLE RESPIRATOR CERTIFIED.

APPLICATIONS
The ARES BBM filtering half mask is a category III P.P.E. in accordance with Regulation (EU) 2016/425 (Law D. No. 17 of 19/02/2019) designed to protect against biological agents and aerosols of different types and airborne particulate matter.
The ARES BBM-certified respirator must be worn when exposed or potentially exposed to infectious agents, as well as when using hazardous substances and mixtures that meet the criteria for classification as carcinogenic or mutagenic category A and 1B.
Applications in the health care sector-hospitals in emergency situations

Application
Scope application

Application in the industry
PROTECTION FROM
BIOLOGICAL AGENTS
| Detail | Features | Benefits |
|---|---|---|

|
Certified against biological agents | Protection against viruses, bacteria, fungi, group 2 and 3 parasites |
| NR | Non-reusable | Single shift use only |
| D | Dolomite dust test | Meets clogging resistance requirements |
| Filter materials | High-performance, lightweight fabrics provide protection in the event of exposure or potential exposure to biological agents | Smaller semi-mask thickness means less breathing resistance and less effort for the operator |
| Adjustable straps and nose wire | Maximum adaptability to all face types | Ensures a good fit to the face even during movement, easy to put on/take off. The pressure exerted by the elastic strings ensures maximum comfort on the neck, face and head for a feeling of safety |
| Elastic strings | Coloured (red) | Protection Level Identification |
| Upper and lower flaps in lightweight materials | Perfect fit to nose and chin | Improves compatibility with spectacles. It improves the field of vision. |
| Foldable | 3-flap design | Easy to store |
| Sealing edge made of soft hypoallergenic material | High grip, collapse-resistant, increased comfort on the face | The half-mask follows the movements of the face. Ideal in hot and humid environments, comfortable in contact with the skin. Improves tightness. |
| Individually packaged | Hygienic | Protection from possible contamination. It allows for convenient storage and distribution. |
| Expiry date and batch printed directly on the device | Safety | Increases worker's awareness of protection. |


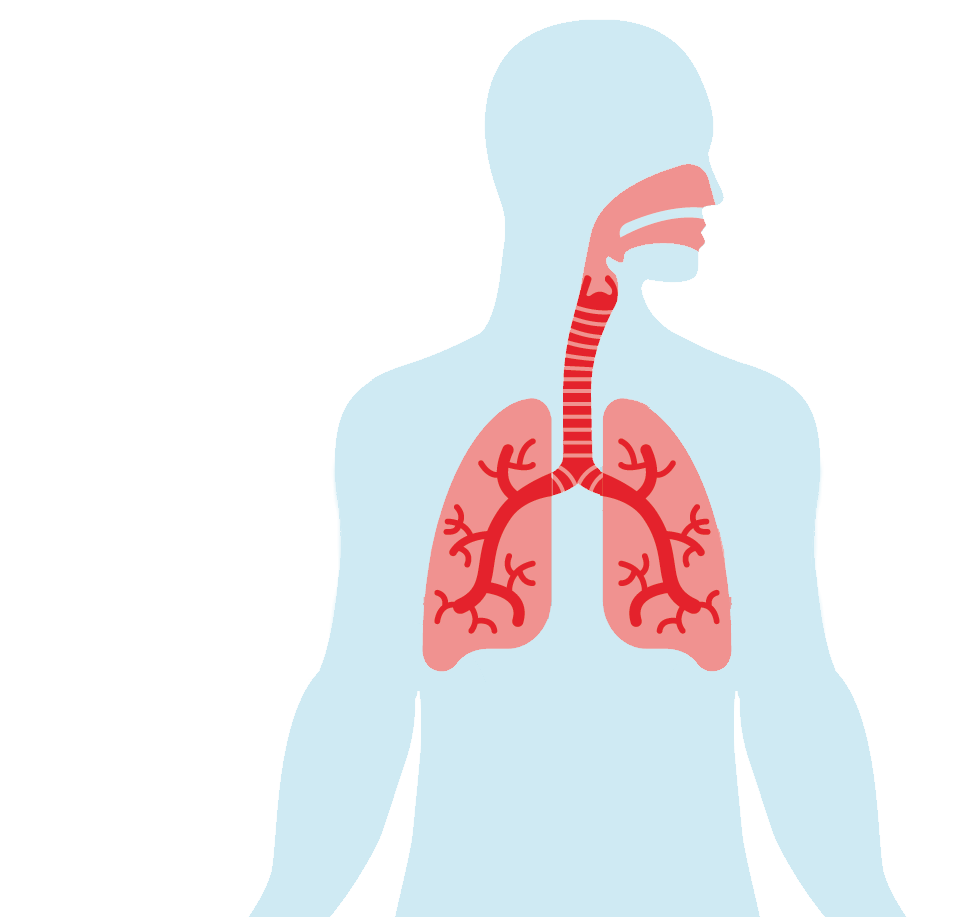
TRANSMISSION ROUTE BY AIR
Comparison
FFP2
-
EN149:2009+A1:2009
(Dust filtering half-masks) -
EN14683
(Medical Devices)
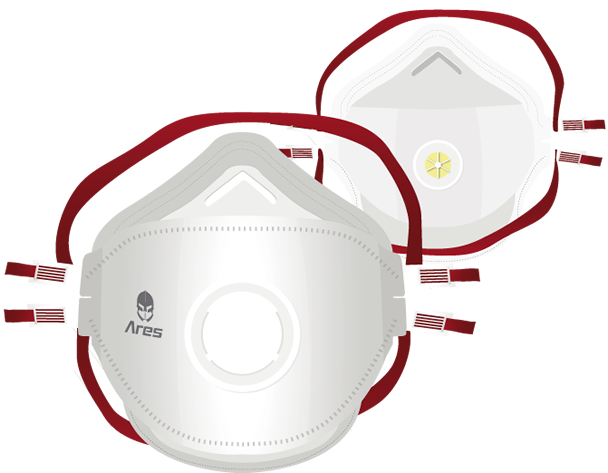
Ares BBM
- Protection from viruses, fungi, parasites and bacteria
-
EN149:2009+A1:2009
(Anti-dust filtering half masks) - Virus protection test performed for each production batch
- Virus protection test performed for each production batch
- Biohazard pictogram
-
EN14683
(Medical Devices)
FFP3
-
EN149:2009+A1:2009
(Certificate for protection against biological agents) -
EN14683
(Anti-dust filtering half-masks)
CERTIFICATIONS
-
Ares BBM filtering half mask is a category III P.P.E. in accordance with Regulation (EU) 2016/425 (Law D. M 17 of 19/02/2019)certified for:
- protection against infectious agents of Group 203 listed in Annex III of Directive 54/2000/EC;
- protection from solid and liquid aerosols in accordance with high technical standard EN 149:200^1:2009 with FFP3 NR D classification.

Each production batch of the ARES BBM – Bio Barrier Mask – filtering half-mask undergoes a third-party filtering efficiency test using concentrated aerosol of MS2 bacteriophage simulating the act of breathing.
A test report is issued for each production batch of the respirator.
Pathogen size

Chlamydia
Ebola Virus

Bacteriophage M13
Vaccinia Virus

Tobacco Mosaic Virus
Adenovirus

Poliovirus
Bacteriophages f2, MS2